A scalable, automated downstream process workflow is fundamental to the industrial-scale manufacture of cell-based therapies. Legacy technologies have often resulted in significant cell product loss, prolonged processing times, and exposure of cells to suboptimal conditions throughout the process – from harvest to final formulation and filling. These inefficiencies can detrimentally affect cell viability, increase batch variability, and ultimately reduce overall product quality and consistency.
Current manufacturing practices in cell harvest, washing, and concentration often involve cell losses of up to 20% due to system inefficiencies and cell damage. Long processing times and prolonged exposure to reagents or buffers can also negatively influence cell health. In the final formulation and filling stages, factors such as temperature fluctuations, residual air, and variability in filling precision introduce additional variables that can hold up batch release. These issues complicate regulatory compliance and increase quality control costs. Collectively, these limitations create an inefficient, labor-intensive workflow that is not sustainable for commercial-scale production.
In response to these challenges, the implementation of an automated downstream process workflow based on closed-system technologies offers distinct advantages. Such a workflow is engineered to streamline each critical processing step, thereby improving cell recovery and preserving cell health while reducing the variability associated with manual operations. For example, automated cell harvest methods utilize equipment specifically designed to minimize cell loss and stress, while integrated washing and concentration systems efficiently remove impurities and meet cell density targets.
Process modeling was performed for a iPSC harvest of 50L volume at a density of 5 × 10^6 cells per mL, relating batch size to processing time and throughput to illustrate a typical downstream workflow - see Figure 1. Cells are harvested and washed through buffer exchange, yielding a preformulated cell concentrate. The concentrated cells are then transitioned into a product cooling phase prior to the formulation phase, during which cryoprotectants such as dimethyl sulfoxide (DMSO) are added prior to aseptic filling into 48 x 50mL cryobags. Figure 1 presents the volume, cell mass balance, and expected processing time for each operation.
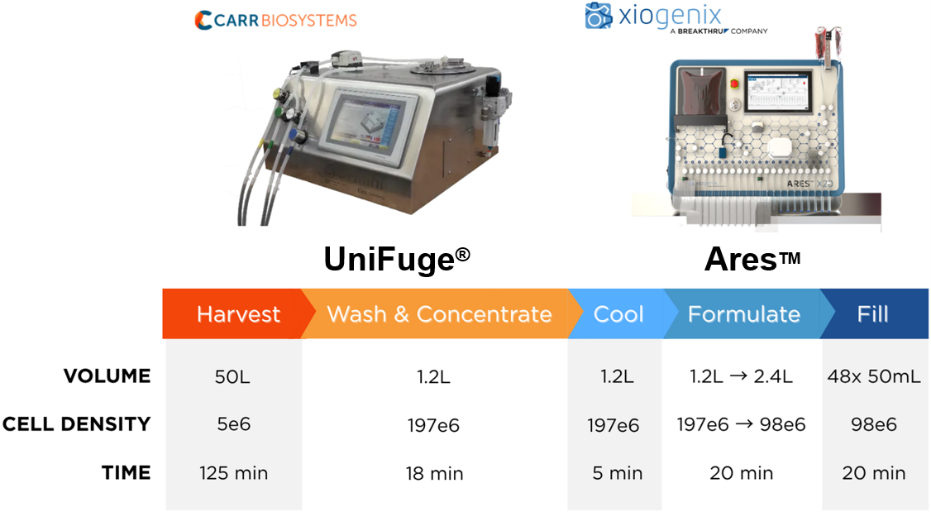
Figure 1 - downstream process workflow.
A critical feature of this workflow is the rapid, closed-system cell harvest, concentration and buffer exchange, which facilitates the transition of cells for final formulation. The concentrated cells are then transferred to an automated formulation and filling platform where an intermediate cooling step is incorporated immediately to preserve cell viability during the temporary hold. This cooling process not only minimizes metabolic activity but also mitigates handling-induced stress, thereby maintaining cell integrity. In addition, an integrated pre-formulation sampling system provides real-time quality control data, ensuring critical process parameters are achieved through final formulation and filling.
During the formulation and fill‐finish phase, the cell product is combined with cryoprotectants to stabilize the cells during cryopreservation, all while being actively homogenized and cooled. The system design emphasizes precision by minimizing residual air and ensuring consistent cell concentrations through consistent source material homogenization and accurate fille volumes, thereby achieving uniform quality across individual doses. The cooling process on the source container is continued through the completion of the filling process to continue the minimization of cell stress. Integrated workflow controls and electronic data capture further enhance process traceability and integrity.
The adoption of an automated, closed-system workflow offers significant economic and regulatory advantages. Automating each processing step reduces product loss and damage, thereby decreasing process variability and increasing overall yield and throughput. Reduced reliance on non-automated processes during early development minimizes downtime and rework during scale-up activities as well as product variability, leading to lower operational costs overall.
In summary, the proposed closed, automated downstream process workflow offers a technical solution to the limitations of current manufacturing methods for allogeneic cell therapies. By integrating automated cell harvest, washing, and concentration, then precision formulation and filling within a closed-system framework, the workflow minimizes cell damage, reduces variability, and enhances overall process efficiency. As the field of cell therapy manufacturing scales, the adoption of such automated, closed-system approaches will be critical for achieving high production yields, reducing costs, and meeting the stringent regulatory standards required for commercial-scale production.
To learn more about the AresTM Fill & Finish system, visit: https://xiogenix.com/cell-and-gene-therapy/
To learn more about the UniFuge® Cell Processing Platform, visit: https://www.carrbiosystems.com/application/cell-therapies